The global pharmaceutical market generates over $1 trillion in annual sales and is built on a complex development process that is often high risk; drugs that look promising during early development can fall flat in clinical trials. M&A activity is often focused on buying drug pipelines to boost R&D spend efficiency and increase time in the coveted patent protection period where the pharma manufacturer has the ability to sell their branded drug without the pressure of generics. While most organizations play by the rules, there are others – both companies and nation states – interested in bypassing or shortcutting the development costs and skipping directly to the production and selling of the drugs.
This article about a pending criminal case against employees from GlaxoSmithKline (GSK) who sought to sell sensitive data around cancer research comes close on the heels of our blog post last week highlighting the challenges of a company in the sustainable energy field losing significant portions of their IP – in this case software source code – to a cyber-espionage campaign backed by the Chinese government. A Tufts University study from 2014 estimated that the average cost to successfully bring a new drug to market is over $2.5b, yet the costs to manufacture (i.e. those incurred by a generic drug supplier) are a small fraction of that figure, typically leading prices to drop once the patent protection period expires. The generic suppliers don’t bear the risk of development and don’t need to cover those costs; on the other hand, the first company to market benefits from a sales period in which they face minimal competition and have significant pricing power. This combination presents significant motive for intellectual property theft between competitors in the pharmaceutical industry; by stealing another competitor’s R&D, a firm can shortcut the costly, high-risk development process and even be the first to bring a drug to market.
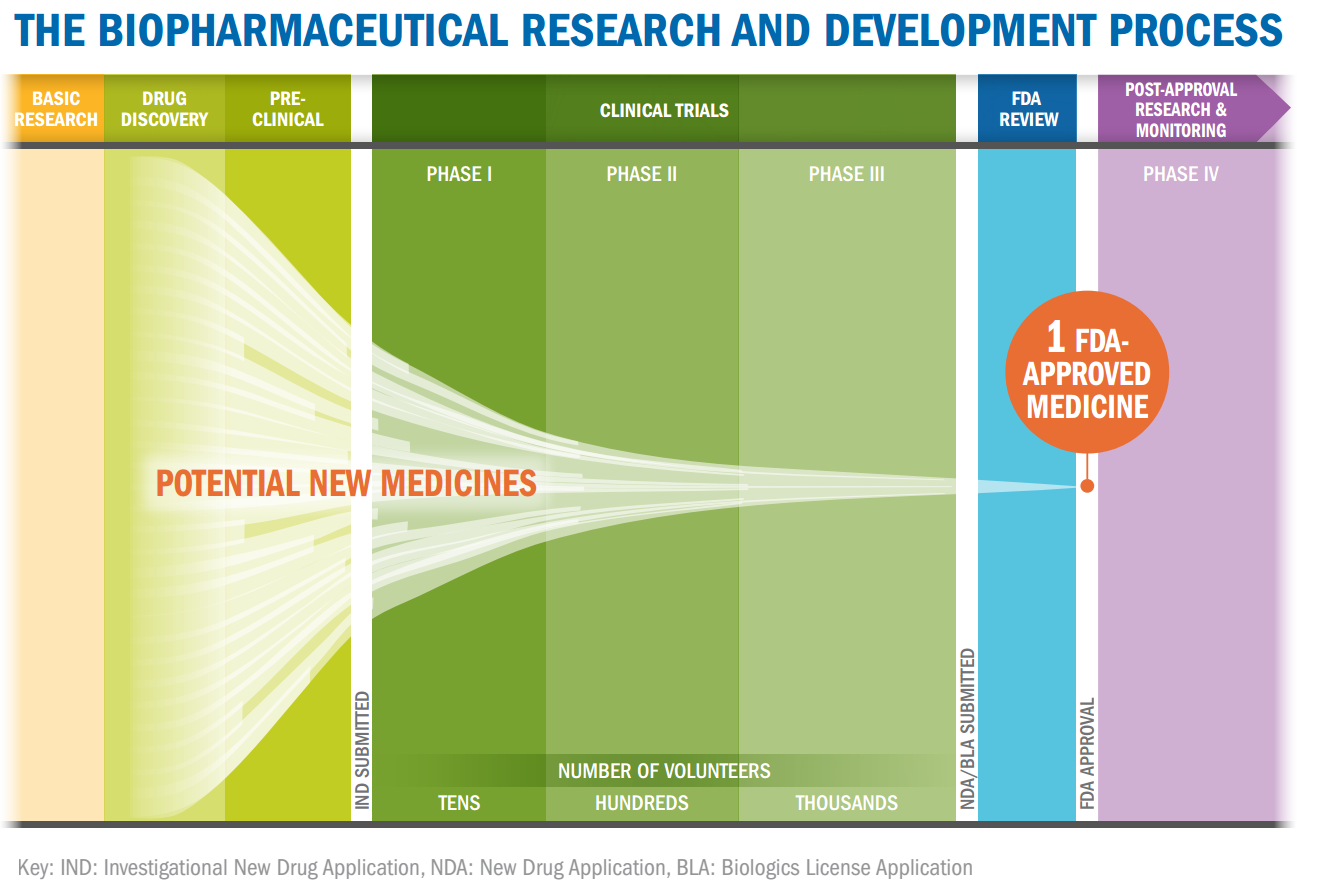
Source: http://www.phrma.org/sites/default/files/pdf/rd_brochure_022307.pdf.
The temptation to skip the risky end of the funnel is what may have spurred the actions of those in the GSK case. While this case is still pending, the details emerging thus far allege that a senior level manager, one that was trained on the importance of protecting the company’s sensitive R&D data, downloaded development details of potential cancer treatment antibodies. According to the charges in the case, that GSK employee worked with a coworker and three outsiders to establish a corporation that would sell the stolen IP to Chinese companies backed by their government.
Cancer research has made great strides in the US in particular, while some regions of the world lag behind and look for ways to catch up. The drug development process can easily eat up 10 years of the 20-year patent protection period, and with the change of US patent law from “first to invent” to “first to file,” pharma companies may be submitting patents earlier to protect themselves from an independent/competitive development. The result is an even shorter period of patent protection before the opportunity for generic market entrants opens up and competitive forces become even stronger.
Research hospitals, pharmaceutical manufacturers, and other biotech companies are all ripe targets for nation states and competitors that are seeking ways to go to market quickly or become more competitive. Organizations who rely on intellectual property as a source of competitive advantage should understand what controls are in place, both technical and administrative, to keep sensitive data where it belongs, and notify infosec managers if these policies are being violated.
Digital Guardian delivers data protection for biotech, pharmaceutical, research hospitals and other IP-centric industries. Would you like to learn more about why 7 of the top 10 patent holders rely on Digital Guardian to protect their data, or how data protection can secure your development process? Read about how Digital Guardian protects protects intellectual property or contact us for a personalized IP protection consultation.
